For Chemistry Professor, Years of Research Yield Breakthrough
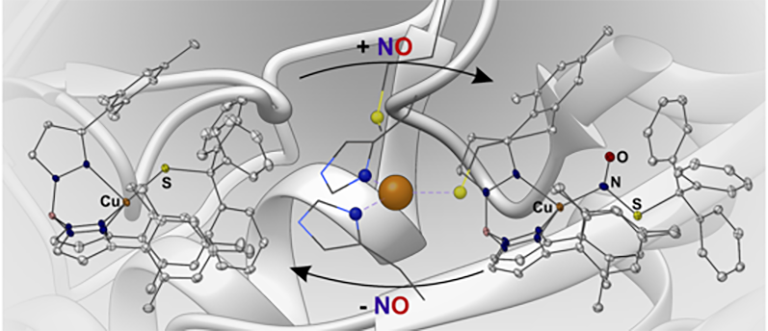
An illustration of the nitric oxide interaction identified by Georgetown College’s Richard D. Vorisek Professor of Chemistry Tim Warren and his collaborators (courtesy Tim Warren).
July 12, 2016 — Twelve years ago, George W. Bush and John Kerry were vying for the presidency. The Detroit Pistons were NBA champions, the secretive internal meetings that would lead to the iPhone were just beginning in Cupertino, Calif. — and Georgetown College chemistry professor Tim Warren was beginning a project that would take him all the way to the spring of 2016.
What kind of research takes this long? Simply a new understanding of one of the most important molecules in cellular communication for everything from soil bacteria to human blood cells: Nitric oxide.
“Sometimes science takes a long time to get right,” Warren said.
Warren, along with Georgetown coauthors Shiyu Zhang and Marie Melzer and Istanbul-based collaborators S. Nermin Sen and Nihan Celebi-Olcum, landed the article “A motif for reversible nitric oxide interactions in metalloenzymes” in Nature Chemistry, the field’s most prestigious journal.
Nitric oxide (NO) is one of the gases that comes out of your car’s tailpipe. It quickly reacts with oxygen to make nitrogen dioxide, the pollutant that turns the air brown in cities like Beijing and Mexico City. But in biochemistry, it’s an important “signaling molecule,” a substance that organisms use to trigger reactions in cells.
In the human body, NO sends the messages that tell blood vessels to open up to enhance blood flow, prevent platelets from aggregating (which causes blood clots), and is partially responsible for the synaptic signals that form nerve impulses.
“Of course, it’s also toxic in larger quantities,” Warren said. “It’s a bit of a Goldilocks molecule — too little and a cell can’t function properly, too much and it’s a biochemical weapon.”
The Georgetown paper represents an important shift in how scientists think about NO, as it identifies a new way this molecule can regulate proteins. It was previously thought that NO, like oxygen, would bond directly to a metal atom in enzymes. But years of painstaking research revealed that NO can go in between a copper-sulfur bond found in a variety of proteins, allowing NO to be interconverted with other biologically important molecules in cellular communication called S-nitrosothiols.
Warren and his team discovered this new bond with the use of a small molecule mimic, a molecule specifically engineered to replicate the structure of a protein’s active sites. This allows them to specifically focus on the function of the active site without potential interference from the thousands of other atoms that comprise a real-life protein.
Biochemists before Warren only knew how NO interacted with copper proteins in broad strokes. The discovery of a specific mechanism by which NO can regulate copper proteins allows new insights into their function in our bodies — controlling iron uptake in the blood, for example — but also in environmental chemistry.
“We basically need to fill the soil with nitrogen to provide enough fertilizer to feed our planet,” Warren said. “Fortunately there are organisms that can decompose fertilizer that is not consumed by crops. One of the enzymes used to return nitrogen back to the atmosphere converts nitrite in the soil to NO, a toxin to these organisms in high amounts. Our findings suggest a self-protection mechanism against NO.”
This in-depth look at the functioning of a molecule central to so many varied and important processes helped the Georgetown paper earn placement — along with a complementary study from the University of Illinois — atop the Nature Chemistry website.
For his part, Warren sees the breakthrough as a teaching experience.
“It’s a redeeming lesson for graduate students to know that it’s OK to tackle something that is extremely challenging,” he said. “With hard work, dedication, perseverance and a fair bit of good luck, you can shoot for very ambitious goals.”
— Patrick Curran