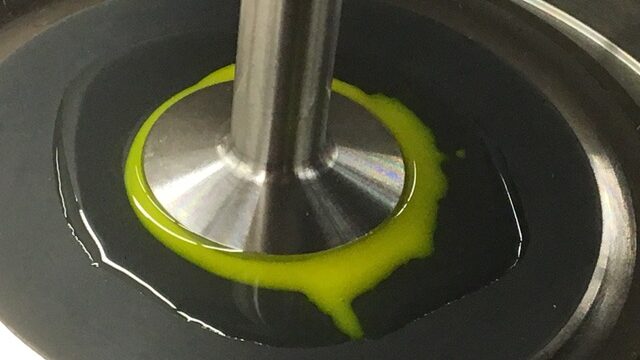
What’s Goo Got to Do with It? Georgetown Physicists Study Fundamental Structure of Flowing Cornstarch-Water Mixture
A team of Georgetown College of Arts and Sciences physicists has captured, in high resolution, video of cornstarch grains flowing through water and interacting to jump between solid and liquid behaviors. For the first time, the motion of the constituent components of oobleck can be seen moving independently, lending insight into the material’s complex structure.
The team’s findings, published in Proceedings of the National Academy of Sciences, should not only delight children of all ages who have played with the substance, but have important practical implications for a wide range of industrial and geophysical applications.
Oobleck, Past and Present
Oobleck is a gooey material composed of particles of a solid substance that are suspended in a liquid without being chemically bonded. Taking its name from the Dr. Seuss book Bartholomew and the Oobleck, the familiar cornstarch and water concoction is the stuff of exciting public science demonstrations and popular science fair projects.
“It has long been known that mixtures of cornstarch and water, like many other mixtures of fluids with solid particles, can transition rapidly from liquid-like to solid when pressure is applied to make part of the suspension flow rapidly,” explains Jeffrey Urbach, one of the paper’s lead authors and a Professor of Physics and Interdisciplinary Chair in Science. “This extra increase in resistance to flow is known as shear thickening, and has been the subject of extensive study for several decades”
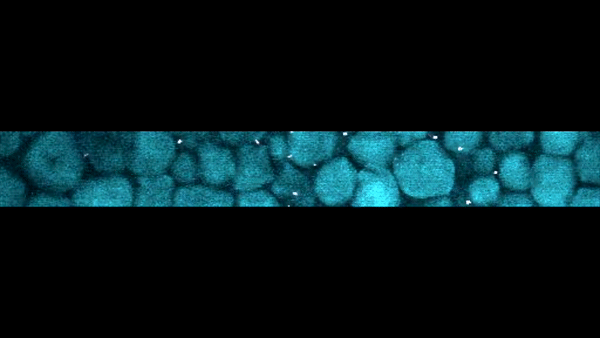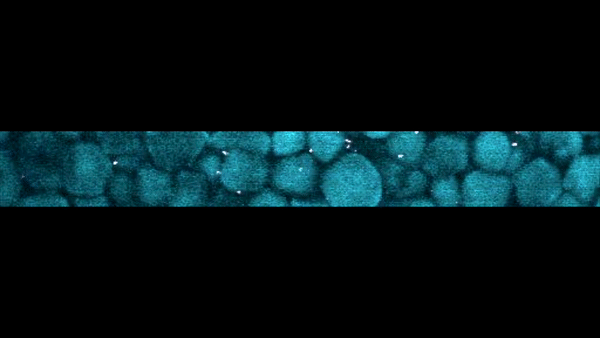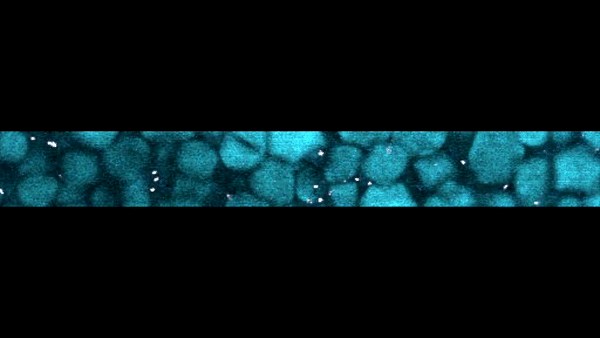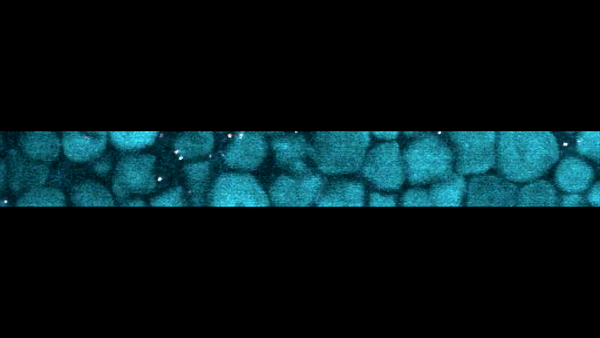
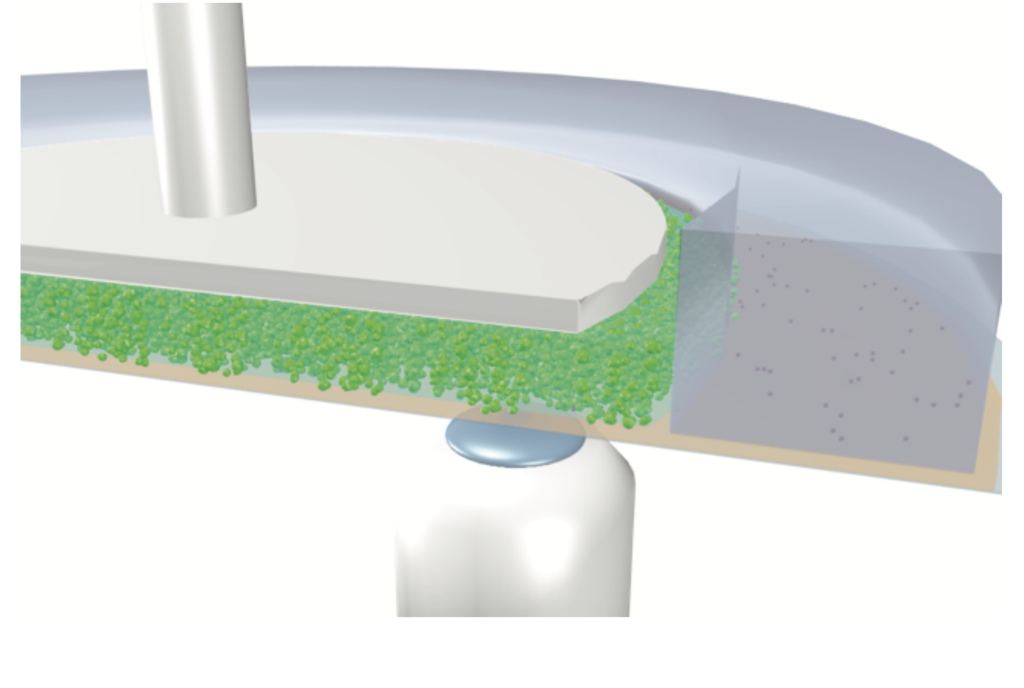
Much about the transition remains mysterious, however, and scientists still don’t have a model good enough to predict where the transition will occur, or design materials that will produce the desired thickening properties. To better understand this process, the research team observed and recorded shear thickening in the mixture using a high-resolution microscope. The oobleck being studied was stirred by a rotating top plate.
The researchers found that, most of the time, water and cornstarch moved together, as expected. But these periods of harmony were interrupted by brief bursts of dramatic differences in flow, with the fluid sometimes going in the complete opposite directions from the suspension as a whole.
“The way the flowing fluid participates when the oobleck particles ‘solidify’ was completely unanticipated, and without experimental expertise that we’ve developed here at Georgetown, we would have never revealed that fundamentally important scientific discovery,” says author Daniel Blair, a Professor in the Department of Physics. “It’s a tour-du-force experiment that our amazing team was able to accomplish.”
The team’s findings show that these flows are associated with stable fronts of jammed material that travel through the suspension at a steady speed, allowing for careful characterization of the motion of the cornstarch particles, the suspending fluid and the forces at the boundary of the suspension.
“Our results illustrate the complex structures associated with shear thickening and show how the fluctuations combine to produce the large forces that result in dramatic increases in viscosity,” explains Urbach. “This research will help point the way towards a complete theory of shear thickening in suspensions like oobleck.”
The research team came entirely from the Hilltop, a collaboration of physicists from the Georgetown College of Arts and Sciences Department of Physics and the Institute for Soft Matter Synthesis and Metrology. Co-authors include Vikram Rathee and Joia Miller.
“We have taken our very technical approach to a hard physics and engineering problem and applied it to a material that everyone that’s visited a science fair or watched a cool YouTube video has explored,” says Blair. “I love telling people that we’ve figured out how oobleck works!”
-by Hayden Frye (C’17)